Wearable Medical Devices: How Flexible Circuits Are Driving Innovation

Wearable medical devices have evolved from simple fitness trackers to sophisticated diagnostic tools that monitor everything from blood glucose levels to cardiac rhythms. This transformation has been driven by advances in miniaturization, materials science, and circuit design, particularly the development of flexible circuit technology. As healthcare shifts toward remote monitoring and personalized medicine, medical device […]
Military Wearable Technology with Next-Level Protection

High-Performance Flexible Circuit Flex Faraday Xtreme™ Military personnel operate in environments where equipment failure isn’t just inconvenient, it’s life-threatening. As defense technology evolves toward sophisticated wearable systems, the circuits powering these devices must withstand extreme conditions while maintaining flawless performance. Nortech Systems’ Flex Faraday Xtreme represents a breakthrough in flexible circuit technology, specifically engineered for […]
Demonstration of Diagnostic Smart Cables in Predictive Maintenance
https://youtu.be/Fxshro3Ns5A?si=vQr9k6JC3Am4WRqc In a world where data speed, power delivery, and reliability are paramount, Nortech is pushing the boundaries of cable technology with its groundbreaking innovations—DDX (Digital Diagnostic Xtreme) and AOX (Active Optical Xtreme). These patented technologies are not just about transmitting data, they’re about transforming how we monitor, maintain, and future-proof our cable infrastructure and […]
The Rise of Near-Shoring in Global Manufacturing

Global manufacturing is entering a new era, shaped by shifting trade policies, technological advancements, and the urgent need for greater supply chain resilience. One trend emerging as a game-changer across industries is near-shoring. This strategic approach has proven to be a powerful asset for companies looking to minimize risks and maximize efficiency. But what exactly […]
AS9100 Certification and Mission Critical Quality

The Role of AS9100 Certification in Quality-Driven Approach When reliability and safety are non-negotiable, quality management must take priority. As a global provider of manufacturing, design, and engineering solutions for mission-critical devices, Nortech Systems places quality at the core of our operations. Our commitment to delivering safe and reliable solutions is underscored by adherence to […]
Overmolded Cables: Injection Molding, and Low-Pressure Molding

Cable assemblies are key components across industries, from medical, aerospace to military applications. Their reliability and protection from external elements can significantly improve the performance of connected systems. Cables with molded connector strain reliefs and junctions provide superior reliability to shrinkable boots and mechanical backshells. Molded assemblies are sealed for life and provide excellent strain […]
Planting Roots for a Sustainable Future with Neighborhood Forest

Since 2023, Nortech has proudly partnered with Neighborhood Forest to bring trees to communities and children across the country. Our commitment to sustainability drives us to support initiatives that create meaningful connections between people and nature. By helping provide access to trees, we aim to foster greener, healthier communities and inspire the next generation to […]
Revolutionary Non-Magnetic Expanded Beam Fiber Optic Cable
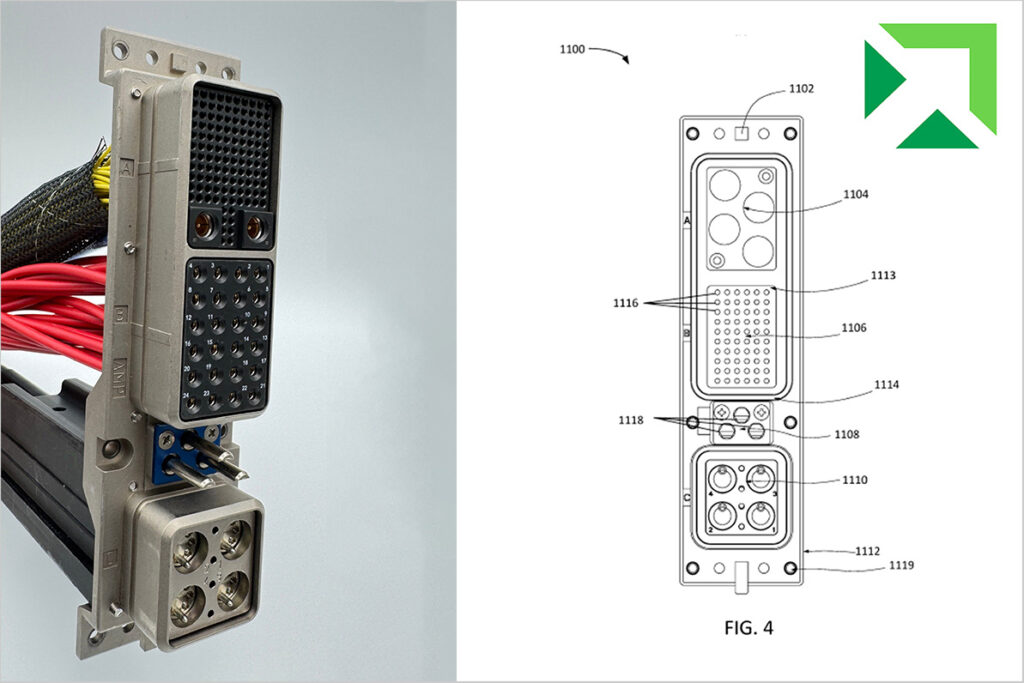
In the ever-evolving world of technology, the demand for reliable, high-speed data transmission is paramount. Nortech’s latest innovation, the non-magnetic expanded beam fiber optic cable patent, introduces a groundbreaking solution. Our technology integrates both optical and electrical elements using expanded beam technology. This patent reimagines the way we think about connectivity, especially in environments where […]
MT Connectors for Fiber Optic Cables | Aerospace & Defense Applications
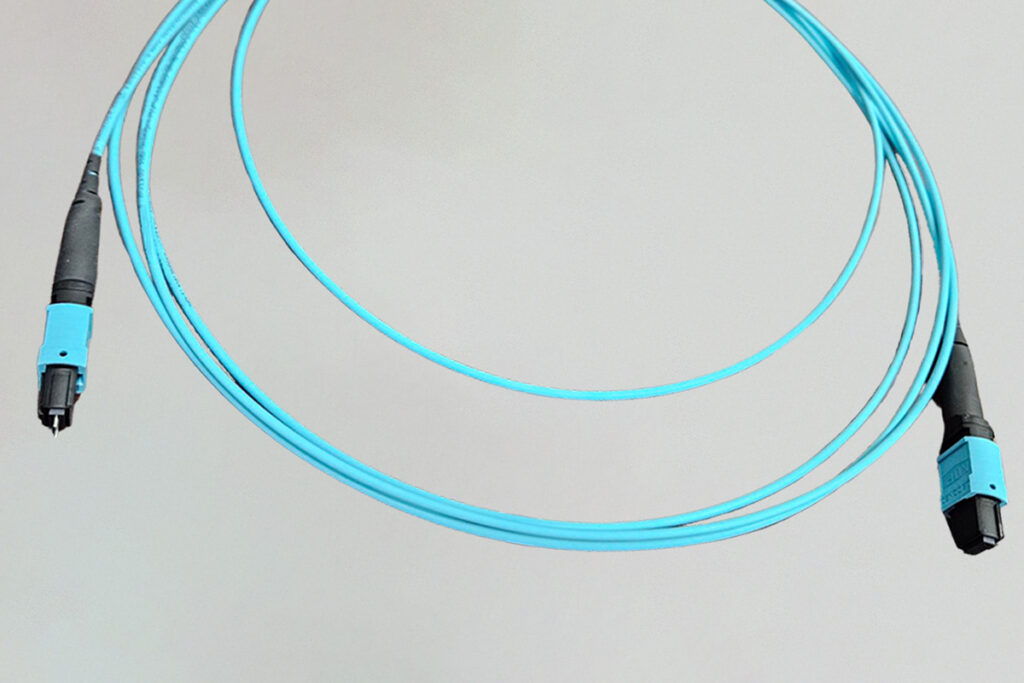
In the aerospace and defense industry, reliable connectivity solutions are paramount. MT connectors for fiber optic cables play a critical role in advanced fiber-optic systems, ensuring high performance and reliability in demanding environments. What Are MT Connectors for Fiber Optic Cables? Definitions: MT Connectors: MT stands for Mechanical Transfer, referring to precise mechanical alignment. They […]
